Patients receive first dose in coronavirus vaccine trials
US researchers gave patients the first shots in a test of an experimental coronavirus vaccine on Monday, leading a worldwide hunt for protection as the pandemic surges.
With careful jabs in the arms of four healthy volunteers, scientists at the Kaiser Permanente Washington Research Institute in Seattle began an anxiously awaited first-stage study of a potential COVID-19 vaccine developed in record time after the new virus exploded out of China and fanned out across the globe.
“We’re team coronavirus now,” Kaiser Permanente study leader Dr Lisa Jackson said on the eve of the experiment.
“Everyone wants to do what they can in this emergency.”
Melbourne researchers map response of coronavirus patient
Meanwhile, the immune responses from one of Australia's first coronavirus patients has been mapped, which could lead to a vaccine.
The immune responses from one of Australia's first coronavirus patients has been mapped, which could lead to a vaccine.
LIVE: Coronavirus news and updates from around the world
Researchers at Melbourne's Peter Doherty Institute for Infection tested at four different points in time blood samples of an otherwise healthy woman in her mid-40s who required hospital admission.
Research fellow Oanh Nguyen says it is the first time broad immune responses to COVID-19 have been reported.
"Three days after the patient was admitted, we saw large populations of several immune cells, which are often a tell-tale sign of recovery during seasonal influenza infection, so we predicted that the patient would recover in three days, which is what happened," Dr Nguyen said.

By dissecting the immune response, the researchers might now be able to find an effective vaccine.
In Brisbane researchers claim they have already found a cure for the coronavirus - an old HIV drug and another drug used to treat malaria.
Both showed promising outcomes when used in COVID-19 patients, according to Professor David Paterson, director of University of Queensland’s Centre for Clinical Research.
Volunteers happy to trial coronavirus vaccine
The Associated Press observed as the Kaiser Permanente Washington Research Institute’s study’s first participant, an operations manager at a small tech company, received the injection in an exam room.
“We all feel so helpless. This is an amazing opportunity for me to do something,” Jennifer Haller, 43, of Seattle said before getting vaccinated.
Her two teenagers “think it’s cool” that she’s taking part in the study.
After the injection, she left the exam room with a big smile: “I’m feeling great.”
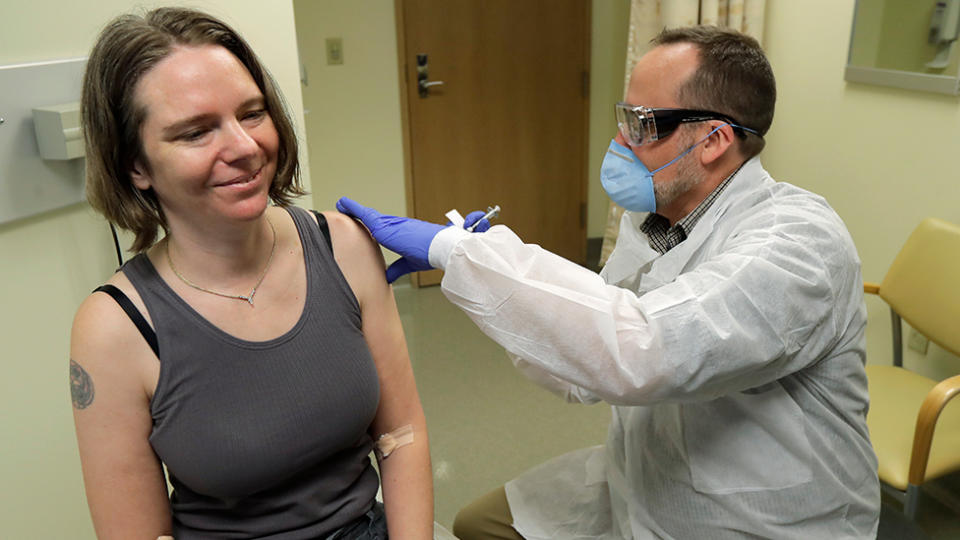
Three others were next in line for a test that will ultimately give 45 volunteers two doses, a month apart.
Monday’s milestone marked just the beginning of a series of studies in people needed to prove whether the shots are safe and could work. Even if the research goes well, a vaccine would not be available for widespread use for 12 to 18 months, said Dr Anthony Fauci of the US National Institutes of Health.
Still, finding a vaccine “is an urgent public health priority,” Fauci said in a statement Monday. The new study “is an important first step toward achieving that goal.”
This vaccine candidate, code-named mRNA-1273, was developed by the NIH and Massachusetts-based biotechnology company Moderna Inc. There’s no chance participants could get infected because the shots do not contain the coronavirus itself.

It’s not the only potential vaccine in the pipeline. Dozens of research groups around the world are racing to create a vaccine against COVID-19. Another candidate, made by Inovio Pharmaceuticals, is expected to begin its own safety study next month in the US, China and South Korea.
The Seattle experiment got underway days after the World Health Organization declared the new virus outbreak a pandemic because of its rapid global spread, which has infected more than 169,000 people and killed more than 6,500.
Vaccine development proceeding at speed
COVID-19 has upended the world’s social and economic fabric since China first identified the virus in January, with broad regions shutting schools and businesses, restricting travel, canceling entertainment and sporting events, and encouraging people to stay away from each other.
Starting what scientists call a first-in-humans study is a momentous occasion for scientists, but Jackson described her team’s mood as “subdued.”
They’ve been working around-the-clock readying the research in a part of the US struck early and hard by the virus.
Still, “going from not even knowing that this virus was out there... to have any vaccine” in testing in about two months is unprecedented, Jackson told the AP.
Some of the study’s carefully chosen healthy volunteers, ages 18 to 55, will get higher dosages than others to test how strong the inoculations should be.

Scientists will check for any side effects and draw blood samples to test if the vaccine is revving up the immune system, looking for encouraging clues like the NIH earlier found in vaccinated mice.
“We don’t know whether this vaccine will induce an immune response or whether it will be safe. That’s why we’re doing a trial,” Jackson stressed. “It’s not at the stage where it would be possible or prudent to give it to the general population.”
Vaccine targeting the protein in the virus
Most of the vaccine research underway globally targets a protein aptly named “spike” that studs the surface of the new coronavirus and lets it invade human cells. Block that protein and people cannot get infected.
Researchers at the NIH copied the section of the virus’ genetic code that contains the instructions for cells to create the spike protein. Moderna encased that “messenger RNA” into a vaccine.
'We need honesty': Government's coronavirus response blasted in fiery Q&A clash
'I feel sad watching this': Wild scenes at Woolworths as elderly queue at dawn
The idea: The body will become a mini-factory, producing some harmless spike protein. When the immune system spots the foreign protein, it will make antibodies to attack — and be primed to react quickly if the person later encounters the real virus.
That’s a much faster way of producing a vaccine than the traditional approach of growing virus in the lab and preparing shots from either killed or weakened versions of it.
- with AP and AAP
Do you have a story tip? Email: newsroomau@yahoonews.com.
You can also follow us on Facebook, Instagram and Twitter and download the Yahoo News app from the App Store or Google Play.




