Safety warning over popular painkiller

A safety alert has been issued for a popular brand of pain relief tablets after it was discovered they were never meant to be put on pharmacy shelves.
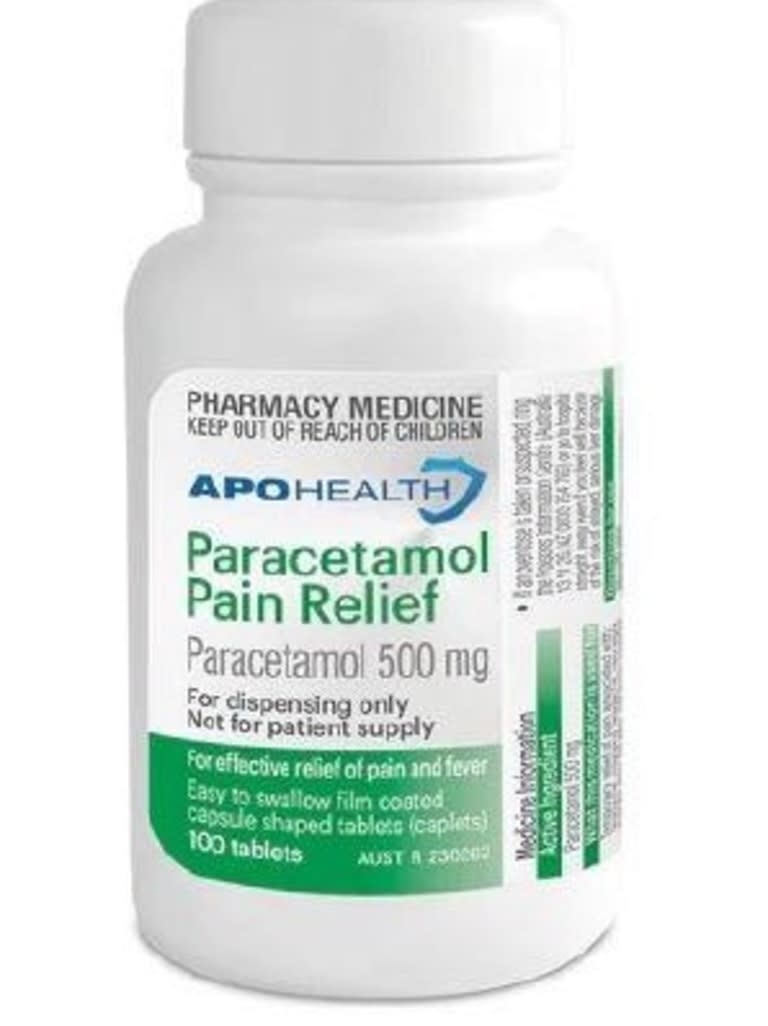
The affected product, Apohealth Paracetamol Pain Relief 500mg film-coated tablets, is sold in a bottle with a green and white label.
According to the Therapeutic Goods Administration, the tablets were never meant to be sold directly to consumers, with the bottle free of any child-resistant packaging.
The bottle’s label explicitly states: “For dispensing only. Not for patient supply.”
The paracetamol product is understood to have been sold in pharmacies and online.

“We are aware that this product may have been sold to some consumers either through physical pharmacies or online stores,” the TGA safety alert said.
“Consumers who may have bought the product should check if they have it and, if so, ensure it is kept out of reach of children.
“There is a potential risk for an accidental overdose should a child be able to access the medicine in the original container.”
It’s recommended the product be returned to the place of purchase if you have concerns about the bottle’s safety.
A separate version of the product, labelled Apohealth Paracetamol Pain Relief 500mg film-coated tablets in blister packaging, was meant for direct supply and is not affected.
If you suspect an overdose has occurred or a child has had an unintended consumption of medicine, contact the Poisons Information Centre on 131 126.


