Ozempic copycats to be banned in Australia
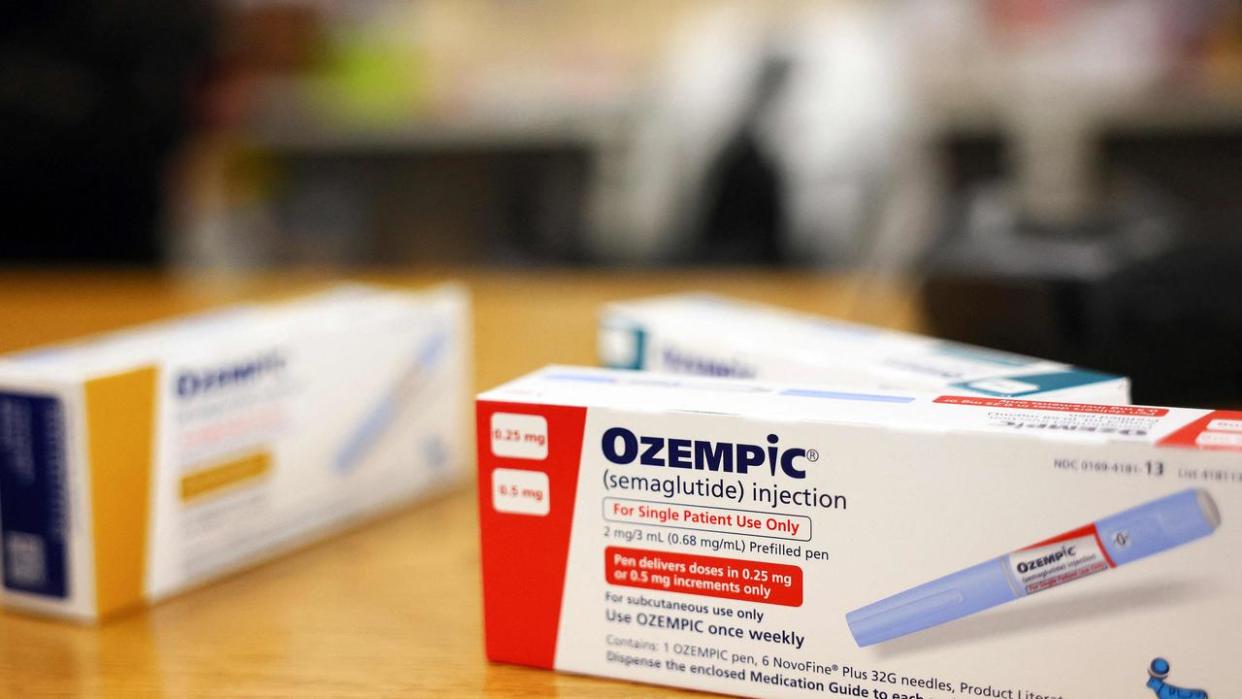
The Australian government has announced that it will be banning replicas of viral diabetes drugs Ozempic and Mounjaro, citing public safety concerns.
A decision made by Health Minister Mark Butler aims to close the loophole that has allowed pharmacies to make and sell copycat versions to about 20,000 Australians.
“This action will protect Australian from harm and save lives,” he said.
Doctors have reported patients experiencing adverse side effects to the off-brand versions, with one patient experiencing tingling in their feet and toes, while another had severe diarrhoea and vomiting.
Other reported side effects include nerve damage, bleeding gums, vomiting blood and rashes, which are believed to be from the replica drugs.

The ban comes after an investigation by Four Corners had uncovered that a registered Australian pharmacist was running an international pharmaceutical racket that manufactured copycat Ozempic and was exporting it to the US illegally.
In times of drug shortages, there is an exemption that allows Australian compounding chemists to reproduce brand name drugs.
This means replicas produced are completely legal, but they aren’t subjected to the same strict safety checks by medicine watchdog Therapeutic Goods Administration (TGA).
Announcing the ban on Wednesday, Mr Butler said it’s difficult to know exactly how many Australians are using the drug and said all stakeholders, apart from the business industry, were supportive of the ban.
“They’re not reporting through Medicare, they’re not reporting through the PBS and that’s really why I was so concerned about it,” he told ABC.
“An exception that was intended to be used on a one-off basis, had essentially been abused to create a large-scale manufacturing and prescribing market that poses very real risks to public safety.”

The ban aims to remove the chemicals in Mounjaro and Ozempic such as glucagon-like peptide-1 receptors agonists (GLP-1RA) from the permitted compounding products list.
This ban will come into effect from October 1, 2024, giving patients time to consult with their GP and plan for alternative treatments.
Those who support the government’s move include the Royal Australian College of General Practitioners, the Pharmacy Board of Australia, Diabetes Australia, the Medical Board of Australia, along with all the state and territories health departments.The drugs that swept through Hollywood and became a popular way for people to lose weight have faced ongoing global shortages, with the TGA maintaining that the shortage will continue throughout the rest of this year.
The shortage is mainly due to health professionals prescribing it off-label, a move that involves prescriptions to treat conditions other than those approved by the TGA. In this case, being prescribed for weight loss over its intended use to treat type-2 diabetes.
The TGA has outlined that it doesn’t have the power “to regulate the clinical decisions of health professionals and is unable to prevent doctors from using their clinical Judgement to prescribe Ozempic for other health conditions.”
Mounjaro’s manufacturer, Eli Lilly announced last year that all strengths of their drug will be limited until August 31, 2024.


