Where Will Moderna Be in 1 Year?
All eyes are on drug manufacturers in 2020 as they race to develop the first coronavirus vaccine. Globally, recorded cases of COVID-19 have surpassed 25 million, with at least 847,000 related deaths worldwide. More than 6 million cases have been reported in the U.S. alone, with at least 183,000 deaths as of Aug. 30.
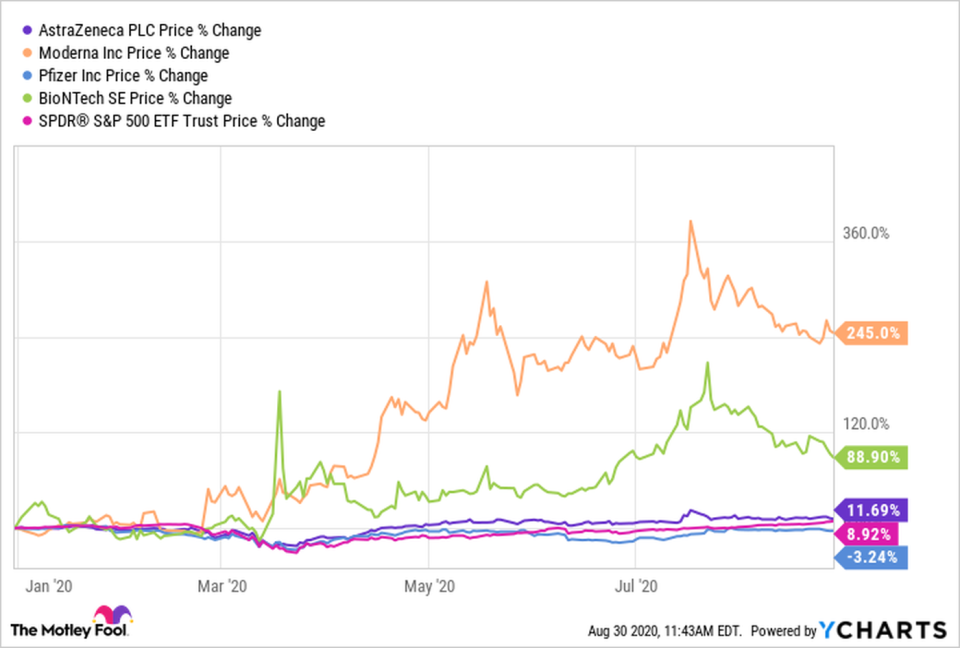
According to the World Health Organization, there are more than 150 novel coronavirus vaccine candidates in development now, with nearly 30 of them in clinical testing. U.S.-based Moderna (NASDAQ: MRNA) reported positive results from the phase 1 trial for its COVID-19 mRNA vaccine in July, and started the phase 3 trial on July 27. It's no surprise that its stock is up 245% year to date, while the S&P 500 has risen just 9%.
But what will happen to Moderna if its late-stage trials aren't successful, or if regulatory approvals for its potential vaccine pose some challenge? Even if it is successful, what does the next year look like for the company?
Moderna's vaccine candidate progress
Moderna's vaccine candidate, mRNA-1273, got a boost in April after it received $483 million in funding from the Biomedical Advanced Research and Development Authority. The urgency of ending the pandemic has led governments to expedite the vaccine development process, which usually takes many years. Moderna recently received an additional $472 million through Operation Warp Speed (OWS, a Trump administration initiative) to support its late-stage trials.
The phase 3 trial for mRNA-1273 will test it at a dosage of 100 micrograms on 30,000 participants to see whether "primary endpoints of prevention of symptomatic COVID-19 disease are achieved in the vaccine-treated group." The phase 1 results published in the New England Journal of Medicine revealed the vaccine dosage was safe, and the phase 2 study is under way.
Moderna is scaling up to produce 500 million to 1 billion doses a year beginning in 2021, with Lonza Group as its production partner. On Aug. 28, management announced that Moderna is in talks to sell 40 million or more doses of its potential vaccine to Japan's Ministry of Health, Labour and Welfare, starting in the first half of 2021. It says it is also in "advanced discussions" with the European Commission to supply Europe with 80 million doses.
Before this, the U.S. government had already pledged more than $1.5 billion for 100 million doses of Moderna's potential vaccine, with the option to buy up to an additional 400 million doses under OWS.

Image source: Getty Images.
What will Moderna look like a year from now?
Moderna's outlook a year from now is highly dependent on how its vaccine candidate fares in late-stage testing.
Management plans to price their potential vaccine at between $32 to $37 per dose for the smaller orders. It has, however, offered a discount to the U.S. government to provide the initial 100 million doses at $15 each -- and why not? The largest funding for its vaccine candidate has come from the U.S. government, after all.
If its vaccine candidate is successful, it could garner huge revenue for Moderna. However, once the pandemic ends, and if the coronavirus does not return, demand for the vaccine could ultimately decline. Transportation and storage of the potential vaccine, which needs to be kept very cold, could also prove unfavorable for the company if competitors make a vaccine that is easier to transport and store.
Keep in mind that in the vaccine development process, many things can go wrong. Vaccines can fail late-stage trials and encounter issues with regulatory approval, and it's always possible that another drugmaker will come out with a successful vaccine first.
Given all that, it's especially important to note that Moderna doesn't have any other approved products on the market. Currently, its other drug candidates are either in phase 1 or 2 trials, or the pre-clinical testing stage. As of Aug. 5, the company said it has "23 mRNA development candidates in its portfolio with 13 in clinical studies."
For now, Moderna is still unprofitable, but its cash position remains robust. As of June 30, it had $3.1 billion in cash, cash equivalents, and investments.
Is Moderna a good investment for the future?
Moderna has tough competition in this race -- Sinopharm, Sinovac Biotech, Pfizer, AstraZeneca, and BioNTech have some of the more advanced candidates -- but its already soaring shares could skyrocket if its vaccine is approved. Shares of Moderna have gained 245% so far this year, while AstraZeneca and BioNTech are up 12% and 89% and Pfizer has declined by 3.2%.
Even if another company surpasses Moderna in the vaccine race, the company can still profit from the other drugs in its pipeline -- but it could be years before they bring in revenue. That makes Moderna a good biotech stock for aggressive investors who believe better rewards come with greater risk.
More From The Motley Fool
Sushree Mohanty has no position in any of the stocks mentioned. The Motley Fool has no position in any of the stocks mentioned. The Motley Fool has a disclosure policy.
Where Will Moderna Be in 1 Year? was originally published by The Motley Fool